Research & Peer Review Studies
Phase 4 Clinical Study of TRMX-3 ® in Boosting MMental & Physical Health
(May 1, 2011 - February 28, 2013).
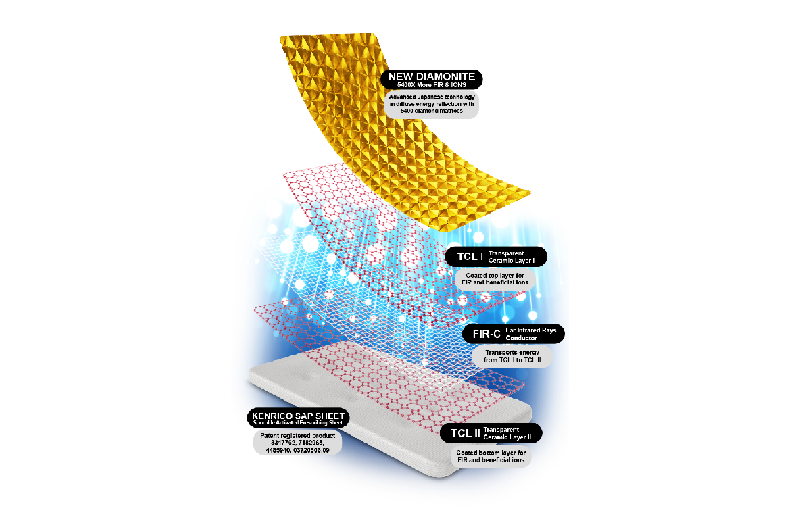
Test article: Kenrico SAP
Sheet Supreme Gold Edition (TRMX-3 ®) with Sporolife
OBJECTIVE
The objectives of this phase 4 human clinical trials is to present data of the long term effects and the differences between placebo to actual cure utilizing the Supreme Gold Edition (TRMX-3 ®) in
boosting mental and physical health and in treating physical injuries. The study, referred to as DEFENSOR II (Determining the EFficacy of the MENtal and PhySical BOosting with spoRopollenin II)with Kenrico's TRMX-3, is a continuance of
phase 3 trials (DEFENSOR I) conducted by the medical team of Woking Football Club and designed to examine the long term safety and
benefits, how the actual treatment compares to the placebos, and how well the treatment works when it is applied more widely.
MATERIAL AND PROTOCOL
Football players and coaches of Woking Football Club (Woking FC) in England were enrolled in the study for ninety-six weeks. A systematic review of clinical trials in which patients were
randomly assigned to placebo was made for the first period of twenty-eight weeks and the third period of twenty weeks. A systematic review of clinical trials in which patients were assigned to actual treatment was
made for the second period of twenty-eight weeks and the fourth period of twenty weeks.
The Supreme Gold Edition (TRMX-3 ®) was selected in the current research study because its positive effects in heavy metals reduction, pain and stress reliefs has been confirmed by the phase 3 clinical trials, the DEFENSOR I, in 2011. In the current human clinical trial, after six weeks of preparation, a group of first team football players and coaches was selected randomly for enrollment in the human clinical trials over the period of ninety-six weeks. A dosage of between 2 (two) to 4 (four) TRMX-3 ® patches was administered daily and continuously for the second period of twenty-eight weeks and the fourth period of twenty weeks under a pre-defined single-blind protocol. A questionnaire must be submitted daily by each participant.
A placebo for the same dosage was administered daily and continuously for the first period of twenty-eight weeks and the third period of twenty weeks under a pre-defined single-blind protocol. The TRMX-3 ® used for the placebo was manufactured without Sporolife.
RESULTS

At the end of the trial, there were statistically significant differences in the performance between the actual treatment with the TRMX-3 ® and the placebo. The actual treatment with the TRMX-3 ® increased the winning ratio to 75% (a 20.16% positive boost for long-term treatment, the second period of twenty-eight weeks) and 55% (13.33% positive boost for short-term treatment, the fourth period of twenty weeks).
The placebo hindered the winning ratio reducing it to 54.84% (29.20% setback for long-term treatment, the first period of twenty-eight weeks) and 41.67% (33.33% setback for short-term treatment, the third period of twenty weeks).
Compared to the placebo, actual treatment with the TRMX-3 ® had significant positive effects on both short-term and long-term use..

The actual treatment with the TRMX-3 ® increased the winning ratio to 20.16% for long-term treatment (second period of twenty-eight weeks) and 13.33% for short-term treatment (fourth period of twenty weeks).
The placebo reduced the winning ratio to 29.20% for longer treatment (first period of twenty-eight weeks) and 33.33% for shorter treatment (third period of twenty weeks).

Questionnaires collected after the actual treatment with the TRMX-3 ® indicated between noticeable to great improvement for the headaches, fatigue, energy & stamina, joint pain, leg aches, body aches, sleep quality, facial skin condition, mental focus & concentration, overall confidence, well-being and moral.
CONCLUSION
The Supreme Gold Edition (TRMX-3 ®) had powerful effects in boosting health when administered as a short-term or a long-term treatment. Although long-term treatment with
TRMX-3 ® had more significant effects on the outcomes, they had slightly better margin in studies compare to short-term treatment. Outside the setting or clinical trials, there is no justification for the use
of placebos as they provide absolutely no health benefits.